How to memorize the periodic table 10X faster - Video 1. Start with the first 20 elements at and then go for t. Atomic Number – Protons, Electrons and Neutrons in Rutherfordium. Rutherfordium is a chemical element with atomic number 104 which means there are 104 protons in its nucleus. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z.
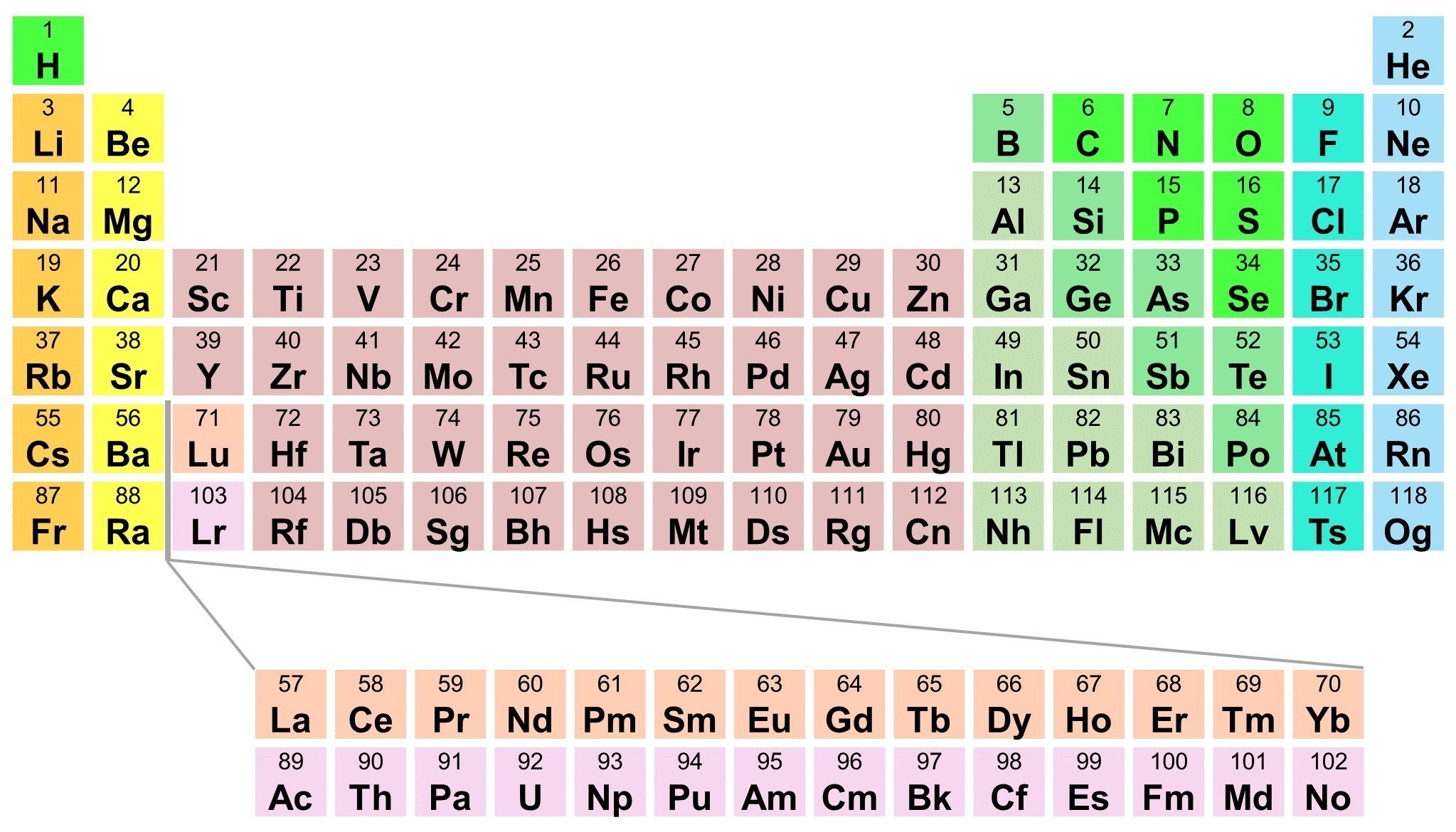
In the periodic table, the elements are listed in order of increasing atomic number Z. It is the Pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. The periodic table of elements arranges all of the known chemical elements in an informative array. Elements are arranged from left to right and top to bottom in order of increasing atomic number. Play this game to review Periodic Table. The vertical (up and down) columns in the Periodic Table are called.

Periodic Number 13
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that determines most of the chemical behavior of an element.
In a periodic table arranged in order of increasing atomic number, elements having similar chemical properties naturally line up in the same column (group). For instance, all of the elements in Group 1A are relatively soft metals, react violently with water, and form 1+ charges; all of the elements in Group 8A are unreactive, monatomic gases at room temperature, etc. In other words, there is a periodic repetition of the properties of the chemical elements with increasing mass.

In the original periodic table published by Dimitri Mendeleev in 1869, the elements were arranged according to increasing atomic mass— at that time, the nucleus had not yet been discovered, and there was no understanding at all of the interior structure of the atom, so atomic mass was the only guide to use. Once the structure of the nucleus was understood, it became clear that it was the atomic number that governed the properties of the elements.
Periodic Table Of Elements Interactive

Symbol
Periodic Number

The period of a number with a repeating decimal is noted with a horizontal line above the sequence of digits that repeats:
(dfrac{2}{13} = 0.153space846space153space846 … = 0.overline{153space846})
Periodic Number 11
Examples
- The number (dfrac{3}{11}) is a periodic number where the period is 27, because: (dfrac{3}{11} = 0.272space727space272 … = 0.overline{27}).
- The number (dfrac{2}{7}) is a periodic number where the period is 285 714, because: (dfrac{2}{7} = 0.285space714space285space714 … = 0.overline{285space714}).
